New Coronavirus Strain in Brazilian Bats Sparks Global Concern — Are We Ready for Another Pandemic?
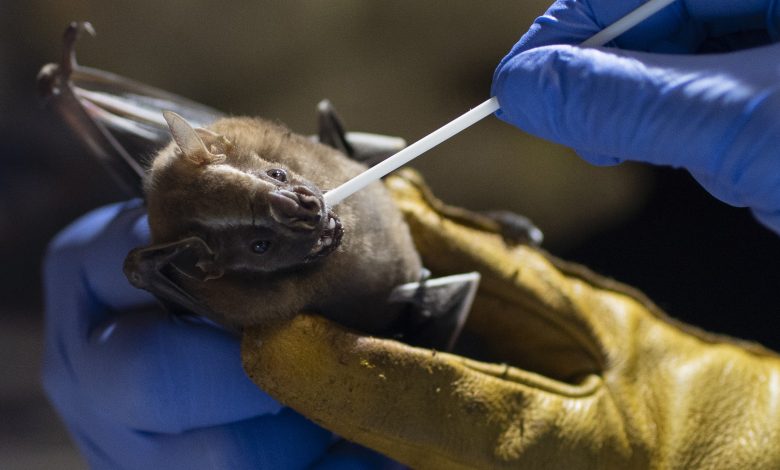
Late in 2025, scientists working in Brazil reported the discovery of a previously unknown coronavirus in local bat populations. The virus—widely referred to in early press coverage as BRZ batCoV—belongs to the betacoronavirus family (the same broad family that includes SARS-CoV-2 and MERS-CoV) and carries genetic features that have attracted particular attention from virologists and public-health experts. While no human cases have been documented, the finding has reignited questions that have become painfully familiar since 2019: how likely is a bat virus to jump into people, how well prepared are we to detect and stop such spillovers, and what practical steps should governments and communities take now to reduce the risk of another pandemic?
Below I break down what we know about the discovery, what it means scientifically, how spillover events happen and why they remain a constant threat, and what a realistic, globally coordinated preparedness plan should look like.
What was found — the essential facts
Researchers sampling wild bats in several Brazilian states detected multiple coronaviruses; one of the viruses stood out because of its genetic similarities to known high-consequence betacoronaviruses. The virus was identified in Pteronotus parnellii (Parnell’s mustached bat), a small insectivorous species distributed across large parts of Latin America. Genome sequencing placed the virus in the betacoronavirus group, and scientists reported a short stretch of the spike protein that resembles a “furin cleavage site” — an enzyme-targeted sequence that, in other coronaviruses, can enhance the virus’s ability to enter animal and human cells. The discovery was published as a preprint and covered broadly in international media while laboratory follow-ups (including secure cell-culture and receptor-binding tests) were being scheduled.
Important caveat: detection of a virus in bats does not mean the virus currently infects — or will imminently infect — humans. Genomic similarity raises a signal of potential risk, not a confirmation of spillover. Lab tests to assess cell entry, host-receptor usage, and animal model infectivity are the critical next steps before any human-risk estimates can be made.
Why scientists are paying attention (and why we shouldn’t panic)
There are two separate reasons scientists flagged this virus as noteworthy:
- Phylogenetic placement. It sits in a branch of betacoronaviruses that includes pathogens known to have caused severe human disease (MERS, SARS). That means it shares evolutionary ancestry with viruses that, on rare occasions, jump species and cause severe outbreaks.
- A molecular feature linked to cell entry. The reported furin-cleavage-like sequence in the spike protein is the type of molecular change that can affect how readily a coronavirus binds to and enters host cells in mammals. In viruses such as SARS-CoV-2, this feature is one factor among many that affected transmissibility and tissue tropism. Finding a similar motif in a wild bat virus is a reason to study it more closely, not proof it will cause human disease.
Put bluntly: this is an important early warning signal, not an immediate alarm bell. Many novel viruses are found in wildlife every year; only a tiny fraction will ever cross into humans and then spread sustainably. But the discovery does matter because it expands the global map of where potentially spillover-capable coronaviruses circulate, and it highlights how under-sampled many regions remain.
How bat viruses jump species — the mechanics of spillover
Spillovers from wildlife to humans typically require a chain of events rather than a single mutation. Key components include:
- Viral compatibility: The virus must be able to bind to a receptor present in human (or intermediate host) cells, or mutate in ways that allow binding. Molecular features like cleavage sites can influence this compatibility, but they’re not the whole story.
- Sufficient exposure: People (or animals that bridge wildlife and humans) must come into contact with viral material. This happens during hunting, butchering, handling of animals, deforestation that brings humans closer to wildlife, or through domesticated animals that act as intermediate amplifiers.
- Opportunity for onward transmission: Even if an individual is infected, the virus must be able to transmit from person to person to start chains that could become outbreaks. Many spillovers are “dead ends” because the virus cannot efficiently transmit among humans.
Ecological and social changes increase both exposure and the evolutionary opportunities for viruses: habitat loss, agricultural expansion, wildlife trade, and climate-driven shifts in species ranges all raise the odds that pathogens cross species barriers.
What the discovery tells us about global surveillance gaps
The Brazilian findings underline a simple but uncomfortable truth: we still detect only a tiny fraction of the viral diversity circulating in wildlife. Field studies that combine ecological sampling with genomic sequencing are accelerating, but coverage is uneven geographically and taxonomically. Many low-resource regions with high biodiversity and intense human–wildlife interaction do not have sustained surveillance programs. That leaves blind spots where viruses with risky features could evolve and circulate unnoticed.
Moreover, sequencing alone isn’t enough. After a genomic signal is found, biosafety-level laboratory work is required to test infectivity and receptor usage in cell cultures and animal models; these facilities are expensive and concentrated in a limited number of institutions worldwide. International collaboration — for rapid, secure follow-up testing — is essential but sometimes slowed by logistic, regulatory, or political hurdles. The Brazilian researchers’ plans to send samples for secure testing abroad exemplify both the strength of scientific networks and the dependence on centralized lab capacity.
Are we ready for another pandemic? Not yet — but we’re better prepared than in 2019
The blunt answer is: we’re better prepared than before COVID-19, but not where we need to be. Strengths since 2019 include:
- Rapid vaccine platforms (mRNA, viral vectors) that can be adapted quickly to new pathogens.
- Improved global surveillance networks and genomic sequencing pipelines that detect & share viral genetic data faster.
- Expanded public-health infrastructure, clinical experience, and therapeutic options (antivirals, monoclonal antibodies) than pre-2019.
Weaknesses remain stark:
- Unequal vaccine and therapeutic production and distribution. Manufacturing bottlenecks and inequitable allocation can leave regions vulnerable.
- Patchy surveillance. The world still lacks uniformly strong systems to detect zoonotic threats early, particularly in biodiversity hotspots.
- Supply-chain and societal fragility. Lockdowns and economic disruption in 2020–22 revealed how unprepared supply chains, health systems, and social safety nets were for prolonged shocks.
- Political will and sustainable funding. Preparedness requires steady investment in non-glamorous public-health functions — workforce, laboratories, data systems — not just crisis spending. Those investments often fade between scares.
So while a discovery such as BRZ batCoV is worrying in principle, the global systems that would limit a spillover’s spread are stronger than in 2019 — if they are used, scaled, and fairly applied.
Practical steps we should take now
Researchers, governments, and communities can take concrete actions to reduce risk and increase readiness.
- Scale targeted surveillance in biodiversity hotspots. Expand ethical, standardized wildlife sampling programs and integrate them with human-health surveillance (One Health approaches). Data should be shared rapidly and transparently with global partners.
- Fund regional high-containment labs and training. Not all follow-up testing should require shipping samples overseas. Building secure BSL-3/4 capacity and trained personnel in undercovered regions shortens response time and empowers local researchers.
- Reduce risky human–wildlife contact. Policy measures that limit deforestation, regulate wildlife trade, improve biosecurity on farms, and support alternative livelihoods for communities that depend on wildlife commerce can lower exposure.
- Strengthen early-warning and data sharing. Nations and institutions should commit to rapid sharing of genomic and epidemiological data, with legal and ethical frameworks that protect contributors while enabling global analysis.
- Invest in flexible countermeasures. Support platforms for rapid vaccine design, stockpiles of broad-spectrum antivirals, and manufacturing scale-up plans that can be activated quickly and equitably.
- Public risk communication and community engagement. Clear, evidence-based messaging reduces fear and counters misinformation. Engaging local communities in surveillance and mitigation builds trust and practical capacity on the ground.
What individuals can do
For the general public, the best actions are not panic but informed caution:
- Avoid handling wild animals and their carcasses.
- Support policies and products that discourage deforestation and irresponsible wildlife trade.
- Follow public-health guidance from trusted authorities if an outbreak is ever suspected.
- Strengthen local community health systems through civic engagement and support for sustained funding.
The role of scientists and the media — be careful with language
Reporting that a “new pandemic virus” has been discovered can be sensational and counterproductive. Accurate language matters: researchers and responsible journalists should emphasize what is known (a virus with some worrisome genetic features was found in bats) and what is unknown (ability to infect humans, transmissibility, clinical severity). Unnecessary alarm can harm bats and conservation efforts — bats play vital roles in ecosystems, and misguided fear has led to persecution of wildlife in past outbreaks, which in turn can worsen disease dynamics.
Bottom line
The discovery of a novel betacoronavirus in Brazilian bats is a scientifically significant event and a timely reminder: the ecological and biological precursors for zoonotic spillovers are widespread, and the world remains vulnerable to new pathogens. Yet discovery alone is not destiny. With coordinated surveillance, rapid laboratory follow-up, investments in regional capacity, and equitable deployment of medical countermeasures, we can reduce the chance that one more animal virus becomes the next global catastrophe. The question “Are we ready?” should not be answered by a single nation or institution; it requires continuous global cooperation, sustainable funding, and political will — not just headlines.




